Resources
CODING & DOCUMENTATION
EDUCATION & EVENTS
ENROLLMENT & CREDENTIALING
 FORMS & MANUALS
FORMS & MANUALS
HEALTHCARE INFORMATION & TECHNOLOGY
 PATIENT HEALTH
PATIENT HEALTH
PHARMACY
 POLICIES & GUIDELINES
POLICIES & GUIDELINES
PRE-SERVICE REVIEW
 PROGRAMS & INITIATIVES
PROGRAMS & INITIATIVES
 PROVIDER NETWORKS & SPECIALTIES
PROVIDER NETWORKS & SPECIALTIES
Blue Advantage® Part B Provider-Administered Drug Precertification Program
Effective August 1, 2023, precertification will be required on Part B provider-administered drugs for your Blue Advantage® patients. If precertification is not obtained, coverage will not be provided under the plan for the provider-administered drugs. Precertification is managed by Prime Therapeutics.
Precertification will be required for the drugs indicated on the Blue Advantage Part B Provider-Administered Precertification Drug List when administered in the following places of service:
- Physician office (POS 11)
- Patient home (POS 12)
- Outpatient facility (POS 19, 22)
Gene therapy/cellular immunotherapy drugs indicated on the drug list require precertification in all places of treatment.
You will be able to initiate or check the status of a precertification for eligible patients by clicking the appropriate “Go” button on the Additional Coverage tab in Eligibility and Benefits.
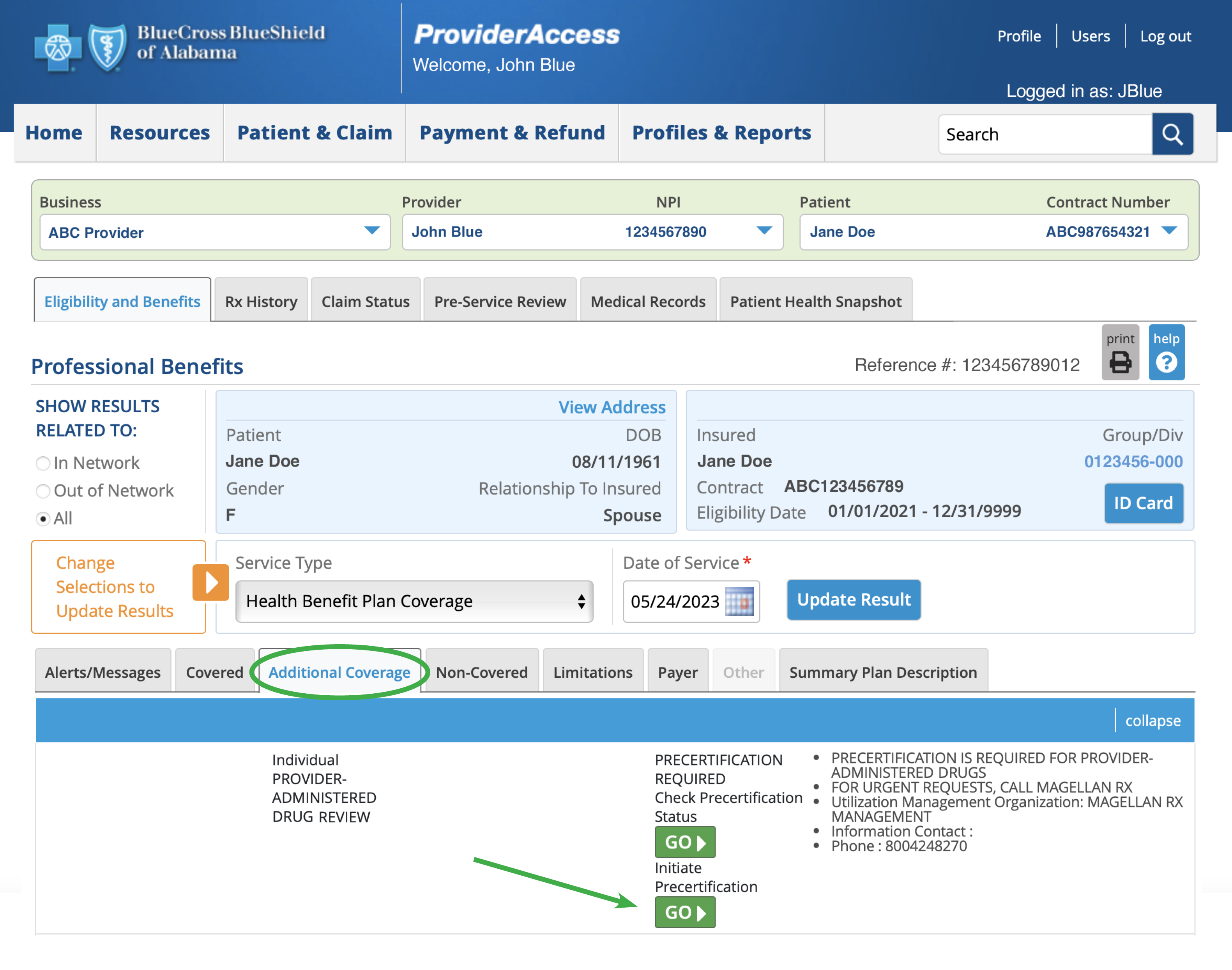
For additional information, review our medical and drug policies at AlabamaBlue.com/Providers/Policies.
Blue Advantage Part B Drug Step Therapy Program
Effective January 1, 2026, certain drugs in the Part B Drug Step Therapy Program are changing from non-preferred to preferred status*. Review the list below to determine which drugs require precertification or are subject to step therapy (i.e., they will be approved with a valid prescription) for your Blue Advantage patients.
| Preferred Products (No Step Therapy Required) |
Non-Preferred Products (Requires Step Therapy) | |
|---|---|---|
|
Rituximab |
|
|
| Trastuzumab |
|
|
| Bevacizumab |
|
|
Prime Therapeutics® LLC, is an independent company contracted by Blue Cross and Blue Shield of Alabama to provide pharmacy benefit management services.